Study Objectives
To evaluate the clinical performance of the SubB2M/CA15-3 test across all stages of Breast Cancer compared to healthy controls and compare the performance of the SubB2M/CA15-3 test to a leading approved CA15-3 test in the same samples in a clinical laboratory setting.
| Table 1: SubB2M/CA15-3 & Comparator CA15-3 Test Performance Summary |
| Breast Cancer All Stages |
SubB2M/CA15-3 |
Comparator |
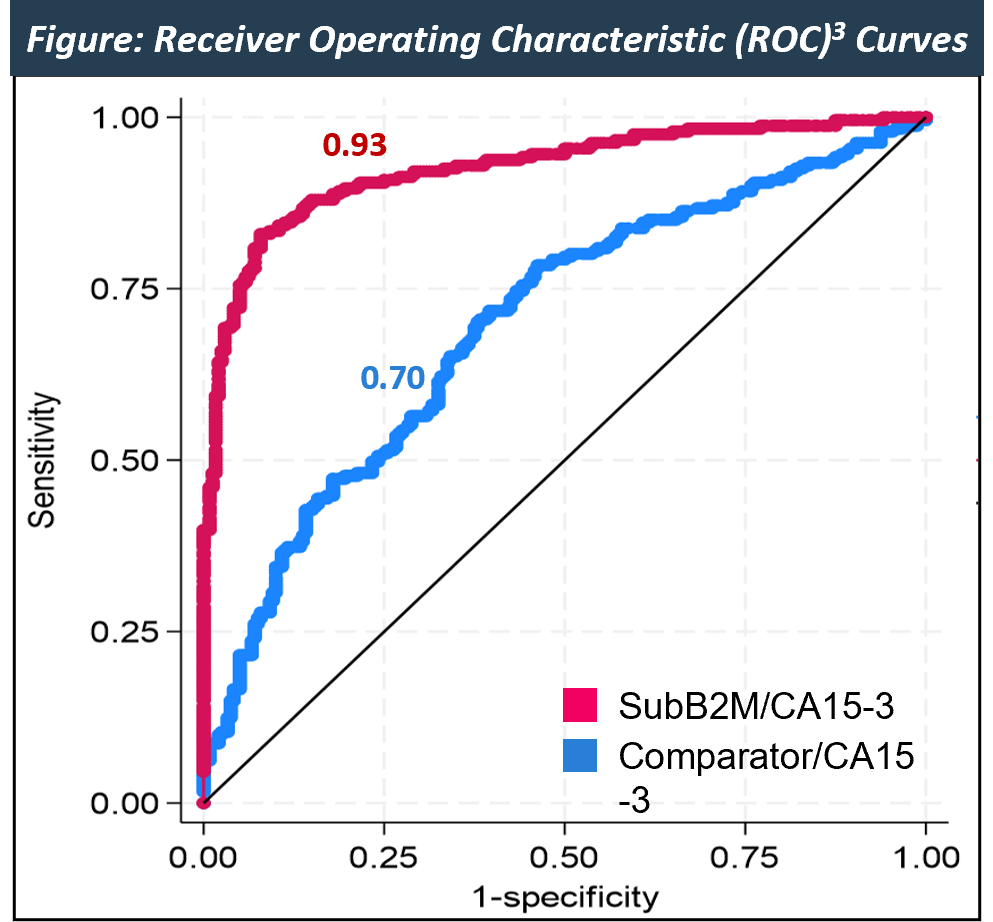
| AUC |
0.93 |
0.70 |
| Sensitivity |
81% |
37% |
| Specificity |
93% |
88% |
| False Negative Rate |
19% |
63% |
| False Positive Rate |
7% |
12% |
| Overall Accuracy |
87% |
63% |
Study Outcomes
SubB2M/CA15-3 breast cancer test provides more accurate detection of breast cancer across all stages, and significantly outperformed a leading approved CA15-3 test.
Data from a 483-sample retrospective case control, clinical validation study demonstrated the SubB2M/CA15-3 breast cancer test significantly outperformed a leading approved CA15-3 test across all states of breast cancer1.
SubB2M/CA15-3 test provides more accurate detection of breast cancer across all cancer stages, demonstrating 81% sensitivity and 93% specificity, compared to a leading approved CA15-3 test2.
1Study performed by ResearchDx under Master Services Agreement (ASX: 5/4/22); 2SubB2M Breast Cancer Test results (ASX: 27/6/23); 3A Receiver Operating Characteristic (ROC) curve is method of estimating the performance of a test to classify samples as diseased or healthy. The area under the ROC curve (AUC) represents how well the test can correctly discriminate between case and control samples; the higher the AUC, the better the test.
Figure 1: Test Sensitivity by Stage @95% Specificity
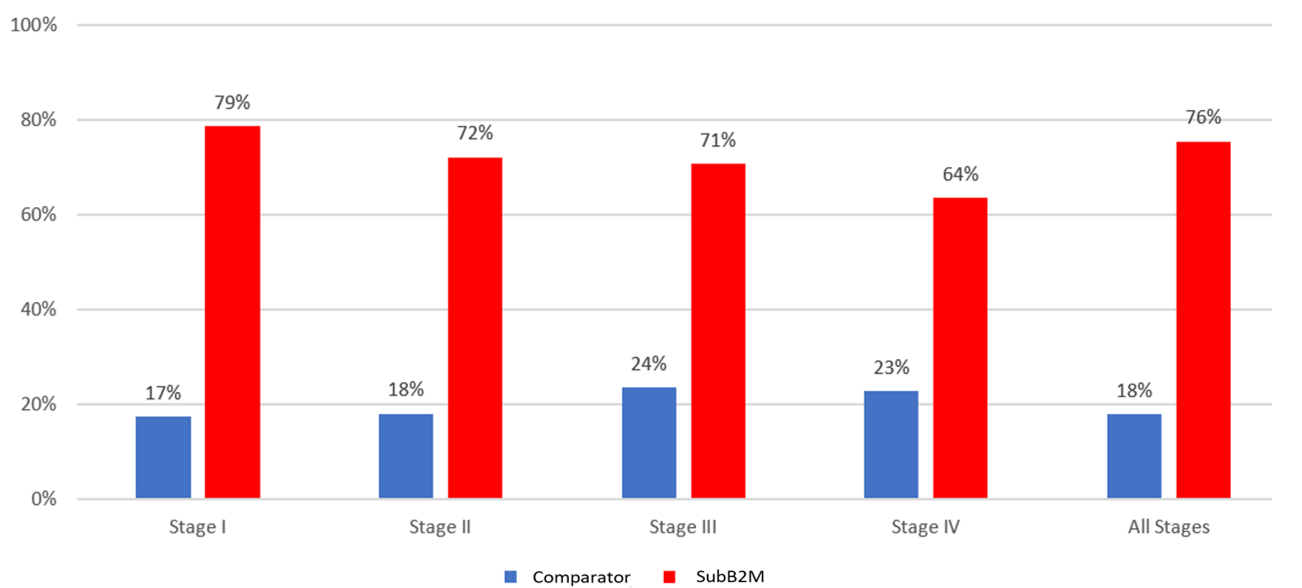 1. SubB2M/CA15-3 clinical validation results (ASX: 27/6/23) ; 2. Breast cancer monitoring study results (ASX: 22/2/24); 3. HR+ = Hormone Receptor, HER2+ = Human Epidermal growth factor Receptor 2 (HER2), Triple-Negative Breast Cancer (TNBC)
1. SubB2M/CA15-3 clinical validation results (ASX: 27/6/23) ; 2. Breast cancer monitoring study results (ASX: 22/2/24); 3. HR+ = Hormone Receptor, HER2+ = Human Epidermal growth factor Receptor 2 (HER2), Triple-Negative Breast Cancer (TNBC)